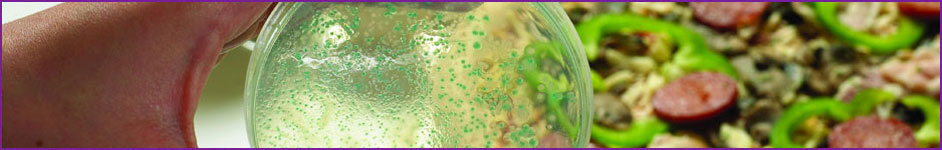
Challenge testing
By Gail Betts - 26 January 2011
Food safety is a major concern for everyone involved in the food industry from producers to policy makers and retailers to consumers, and it is imperative that all precautions are taken to safeguard consumers' health.
Microbiological challenge testing is the laboratory simulation of what can happen to a food product during distribution and subsequent handling if it were to be contaminated with a micro-organism.
At Campden BRI, we have witnessed in recent years an increasing interest in the use of challenge testing procedures to assess the shelf-life of food products. This has been driven by a number of factors including: legislative pressures from EU Directives on the issue of food safety, the increasing requirement for compliance with regards to due diligence protocols, commercial pressures from food manufacturers looking to extend shelf-life to reduce wastage and maximise revenues, and changes to consumer eating patterns in favour of convenience foods.
In light of the increased focus on microbiological challenge testing, the European Commission recently proposed new guidelines for the food industry. These new guidelines, while rigorous are designed for expert laboratories and focus solely on Listeria monocytogenes.
For those companies wishing to use challenge testing with other micro-organisms, or seeking a more user-friendly method, we have recently published guidance that they can adopt.
Unlike the EC guidelines, our procedures test pathogens such as Listeria monocytogenes in ready-to-eat foods, Clostridium botulinum in chilled, modified atmosphere packaged (MAP) foods, and specific spoilage organisms, such as yeasts and moulds, in acidic products.
The Campden BRI method of challenge testing goes further by covering all potential micro-organisms that companies may wish to consider. It also advocates the use of samples taken from a minimum of one batch with three to five replicates, with less onus on achieving precise measurements of target inoculum level. This simplifies the challenge testing process, keeping costs down while still ensuring food safety legislative requirements are strictly adhered to.
That said, while microbiological challenge testing is a highly effective way of simulating in a laboratory what can happen to a product during distribution and subsequent handling if it were to be contaminated with a micro-organism, it is important that it is not undertaken just for the sake of it.
Food manufacturers have the option to use a microbial growth prediction service, based on mathematical growth models. These predictive models are by far the quickest method, allowing cost effective testing of different 'what if' scenarios when reformulating or developing new products, to predict the levels of microbial growth.
This can effectively offer food producers a screening service. If this shows that there is potential for the spoilage of the product due to pathogens, challenge testing can then be carried out to establish whether micro-organisms such as Listeria monocytogenes can grow in the event of contamination and the rate of growth.
Campden BRI's guideline (Guideline 63 - Challenge testing protocols for assessing the safety and quality of food and drink) contains the necessary information for companies wishing to follow a standardised protocol for challenge testing their products.
*This blog was first published in Food & Drink Digital

About Gail Betts
Dr Gail Betts has worked at Campden BRI for over 40 years. She started working in our Thermal Microbiology group in 1984 and was part of the research team that produced the data on the heat resistance of Listeria monocytogenes and Clostridium botulinum that forms the basis of industry accepted heat processes of 70°C for 2 minutes or 90°C/10minutes aimed at producing safe chilled food products. Gail then moved on to manage the Processing, Preservation and Spoilage section within the Microbiology department where she studied predictive food microbiology, the growth and survival of spoilage organisms and food pathogens and use of traditional and novel food preservation systems. She did her industrial PhD at Surrey University during this time on the predictive microbiology of meat products.
Gail has written many guidance documents on shelf-life protocols and challenge testing and has been a member of the European Working group that produced the technical guidance document for conducting shelf-life studies on Listeria monocytogenes in ready-to-eat foods. She was also part of the ISO working group that produced the international standard on challenge testing. Gail is a member of the MicroVal Technical Committee which is responsible for validation of alternative microbiological test methods and regularly attends meetings of the Advisory Committee on the Microbiological Safety of Foods as an invited member of the public.
Gail became the Head of Microbiology at Campden BRI in July 2021 and now manages over 50 experts and technical staff focussing on molecular microbiology and methods, emerging microbiology and viruses, microbiological safety and spoilage, industrial process microbiology, brewing microbiology and microbial risk assessment.
Working at Campden BRI has given Gail insights into many areas of the food, drink and allied industries. She has helped many companies to develop new products, evaluate new processing options, optimise packaging solutions and ensure the safety of chilled, ambient and frozen food products. She is still as passionate as ever about translating scientific knowledge into practical solutions for the food industry within the UK and globally.

