
Nutrition policy developments: An update on the United States
9 June 2025 | Millie Preston, Regulatory Affairs Advisor and Lewis Wallis, Regulatory Affairs Advisor,
At the Conference on Hunger, Nutrition, and Health on September 28, 2022, the White House released a National Strategy to end hunger and increase healthy eating and physical activity by 2030, so that fewer consumers experience diet-related diseases like type 2 diabetes, obesity and hypertension. 2024 was a busy year for subsequent nutrition policy developments in the United States, with three major developments from the federal government:
1. Dietary Guidelines for Americans (Health and Human Services (HHS) & United States Department of Agriculture) (USDA)
The Dietary Guidelines for Americans have evolved over time, building on evidence-based recommendations that adapt as science develops. While many of its recommendations have remained relatively consistent, the 2025-2030 Dietary Guidelines Report emphasises the importance of maintaining nutrient-dense diets tailored to individual preferences, cultural traditions, and budgetary considerations. It also takes the dietary patterns approach further by encouraging healthy eating habits, from birth to older adulthood, recognising that early dietary choices influence long-term health outcomes. The recommendations are tailored to each life stage and emphasise how they should be maintained throughout life, including, for the first time since 1985, specific guidance for infants and toddlers.
The guidelines serve as the foundation for federal nutrition policies (e.g. SNAP, WIC) and guide nutrition education initiatives (e.g. MyPlate: the federal symbol for healthy eating) by policymakers, healthcare providers, and educators. They are updated every five years and development includes five key steps: 1) identify the scientific questions 2) appoint the Advisory Committee 3) review the scientific evidence 4) develop the dietary guidelines 5) implement the dietary guidelines.
UK comparison: The Eatwell Guide has similarly evolved over time, reflecting evidence-based dietary recommendations that adapt as scientific understanding progresses. It is used as a policy tool used to define government recommendations on eating healthily and achieving a balanced diet.
2. The definition of ‘healthy’ (FDA)
A final rule published in December 2024 updated the conditions of use for the voluntary term “healthy” on product labels.
The original definition was set in 1994 with thresholds for vitamin A, vitamin C, calcium, iron, protein and fibre, and limits for total fat, saturated fat, cholesterol and sodium. The new definition requires products to have a set amount of food from at least one food group as recommended by the dietary guidelines (e.g. grains, dairy, protein, fruits and vegetables), and restricts products that exceed set limits for sodium, added sugar and saturated fat from using the claim.
The FDA is also exploring the development of a “healthy” symbol to allow manufacturers to promote foods that meet the definition.
UK comparison: The term ‘healthy’ is considered a non-specific, general health claim that would need to be accompanied by a relevant, authorised, specific health claim.
3. Front-of-package nutrition labelling (FDA)
In the National Strategy, there was a recommendation to propose a standardised front-of-package nutrition label (FOPNL) to help consumers quickly and easily identify foods that can help them build a healthy dietary pattern. The Transparency, Readability, Understandability, Truth, and Helpfulness in Labeling Act of 2023 (the TRUTH in Labeling Act) directed the U.S. Food and Drug Administration (FDA) to develop front-of-package labels for foods and beverages sold in the United States.
As such, the FDA has been conducting consumer research and testing several schemes: a monochrome information label, interpretive ‘Low/Medium/High’ label (with/without DVs), warning-type label (with/without DVs). Whether the scheme should focus on only including nutrients to limit (similar to warning labels in Latin America ) or those nutrients to encourage (similar to UK traffic lights ) remains an open question.
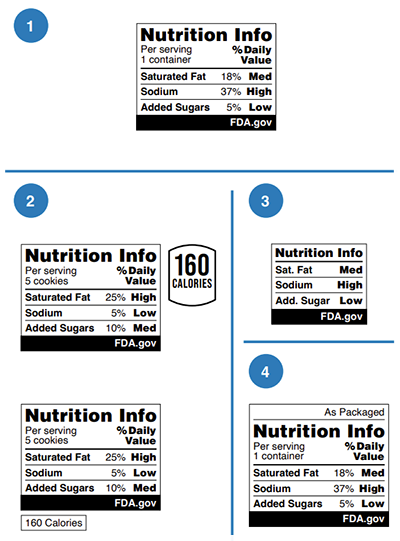
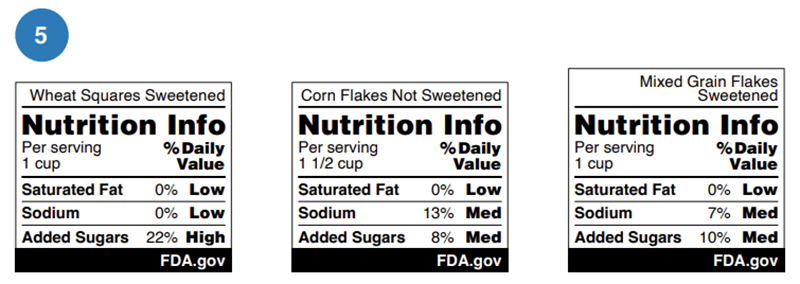
Nutrition examples from U.S. Food & Drug Administration (FDA)
On 14 January 2025, FDA proposed requiring a front-of-package nutrition label, known as the “Nutrition Info” box, for most packaged foods. The “Nutrition Info” box would indicate the levels of saturated fat, sodium and added sugar as either "Low," "Med," or "High". The FDA's research found that a black and white design with percent Daily Value was most effective for consumers.
Public comments are open until 16 May 2025.
If finalised, the rule would require food manufacturers to comply within three years, and smaller businesses within four years. The rule would apply to all food covered under the current nutrition labelling of food regulation for food that is marketed for people ages 4 and older (unless a specific exemption applies). This initiative is part of the FDA's efforts to combat chronic diseases, which are major drivers of healthcare costs in the U.S.
UK comparison: The voluntary UK Front-of-Pack Nutrition Label (i.e. traffic light scheme) was first published in 2013 and assigns colour-coding to four nutrients (fat, saturated fat, sugar, salt) based on nutrient thresholds.
How can we help?
As experts in food compliance and regulatory consultancy, we are fully equipped to support manufacturers and producers looking to sell their products in the US market ahead of these proposed potential changes.
Our services include assisting with regulatory compliance, guiding you through label development/review, assessing the nutrient profile of your products and ensuring nutrition information is formatted correctly. We also offer expertise in front-of-pack nutrition labelling systems (e.g. UK traffic light labelling, Nutri-Score, black octagon warning labels), helping you align with international regulatory requirements and enhancing consumer trust. We are committed to helping you navigate these changes smoothly and ensure your products are market-ready and fully compliant.

About Lewis Wallis
Lewis is a Regulatory and Nutrition Affairs Advisor in the Global Regulatory Affairs team at Campden BRI and has previous industry experience from working for a large multi-national company. He has contributed to and written material for a variety of outputs including research publications, technical reports, food law updates, blog articles, white papers, book chapters, eBooks and guidance documents. Lewis presents on Campden BRI courses and at a range of industry and academic events on the topics of High Fat Sugar Salt (HFSS) legislation, ultra-processed foods, and front-of-pack nutrition labelling.
He is a member of the IFST Food Regulatory Special Interest Group that work to host thought-provoking discussion workshops which feature experts presenting on the latest regulatory hot topics.
Alongside his current role, Lewis is a Postgraduate Researcher at University of Leeds and draws upon his regulatory expertise to conduct research at the intersection of food legislation and consumer behaviour, particularly focusing on measures designed to promote healthier and more sustainable food choices within digital food environments (e.g. online retail, meal delivery apps, social media). He has conducted research on the implementation of HFSS restrictions within online retail and his work involves the application of nutrient profiling models and processed food classification systems to products promoted and sold in digital settings.
Want advice?
Got a question directly related to developments in the US market, or any wider concerns around Front-of-Pack Nutrition Labelling (FoPNL) schemes?
Like to recap on some other key regulatory issues?
Why not take a look at our most recent collection of articles relating to regulations around food.







