
Legislation on country of origin or place of provenance for primary ingredients
30 March 2020 | Ellie Rathkey, Lorna Yates and Helen Arrowsmith, Campden BRI
Background
Legislation relating to the provision of information on the origin of primary ingredients applies from 1 April 2020. This white paper provides a summary of when the requirement is triggered and includes a flow diagram to help businesses decide whether they need to provide a statement on primary ingredient origin.
Requirements for providing information on the country of origin or place of provenance of food and drink products and ingredients are detailed in Article 26 of the Food Information to Consumers Regulation (EU) No. 1169/2011 (FIC).
The FIC states, in Article 26(2), that there are two circumstances where provision of origin information is mandatory;
- Where failure to indicate this might mislead the consumer as to the true country of origin or place of provenance of the food; and
- For meat sold as such (fresh, chilled or frozen) of swine, sheep or goats and poultry (Gallus domesticus, ducks, geese, turkeys and guinea fowls)
It is also mandatory to state the origin of foods subject to product specific controls, such as minced meat, eggs, fruit and vegetables, honey, olive oil, beef and veal.
Point a) above means it is a legal requirement to correct any implied origin made on a product through statements, pictorial presentation, symbols or terms, if the origin given is not a true reflection of where the product is from. For example, a croissant displaying a picture of the Eiffel Tower on the label would need to include a statement that it was made in the UK, if this was the case.
Please note: It is not mandatory under current or new legislation to provide an origin statement for most food products, though such statements are often voluntarily provided.
What is changing?
Commission Implementing Regulation (EU) No. 2018/775 establishes rules and requirements for the application of Article 26(3) of the FIC, regarding the indication of the origin of the primary ingredient of a food.
Regulation (EU) No. 2018/775 requires the country of origin or place of provenance of the primary ingredient in a food to be provided where the origin of the primary ingredient is different from an “origin indication” given on a food label.
‘Primary ingredient’ means an ingredient or ingredients of a food that represent more than 50% of that food or which are usually associated with the name of the food by the consumer and for which in most cases a quantitative indication is required.
An “origin indication” can either be the mandatory declaration referred to above, in accordance with Article 26(2) of the FIC, or a voluntarily indication, in the form of statements, pictorial presentations or symbols.
Do you need to provide a statement on primary ingredient origin?
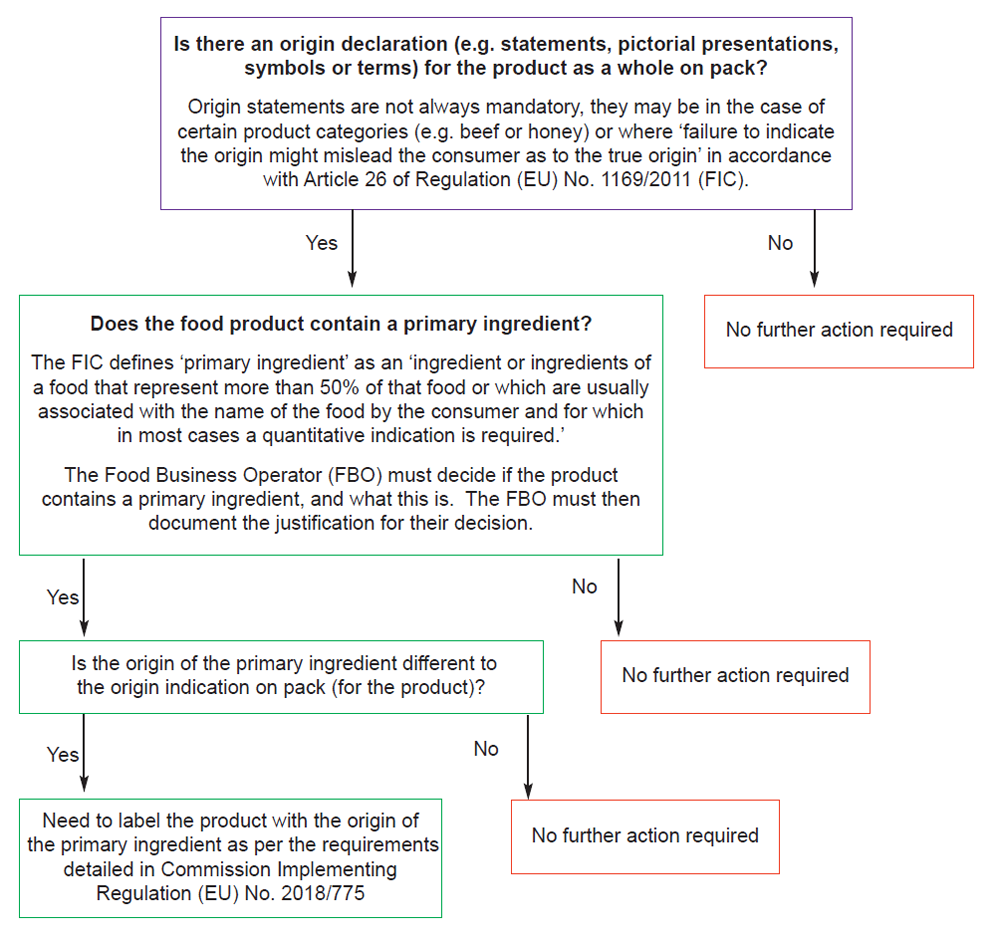
Figure 1. Legislation flow chart
Please note: The requirement to state the country of origin or place of provenance of the primary ingredient is not triggered;
- if the origin of the product as a whole is not stated/implied; or
- if the origin of the primary ingredient is not different to that of the product as a whole; or
- if there is no primary ingredient.
The following are examples of statements that are believed to be generally understood by consumers as “origin indications” and therefore, in principle, should be seen as indicating the country of origin or place of provenance of a food. Use of these statements may then trigger the requirement to provide the origin of the primary ingredient of the food but, only where the origin of the primary ingredient is different to that indicated by the statement.
- ‘made in …’
- ‘manufactured in …’
- ‘produced in …’
In contrast, the following statements are not considered as “origin indications”. Their use, therefore, does not trigger the requirement to provide origin information for the primary ingredient.
- ‘packed in …’
- ‘made by X for Y’
- ‘produced by X for Y’
When does the primary ingredient origin legislation apply?
When the UK left the EU on 31 January 2020, under the Withdrawal Agreement we entered a Transition Period during which EU rules continue to apply in the UK, until 31 December 2020. The date of application of Commission Implementing Regulation (EU) No. 2018/775 is 1 April 2020; this application date applies throughout the EU Member States and the UK.
To make provision for penalties and enforcement of Commission Implementing Regulation (EU) No. 2018/775 national legislation is required in the devolved nations.
What guidance is available?
European Commission (30 January 2020) NOTICE on the application of the provisions of Article 26(3) of Regulation (EU) No 1169/2011 with regard to the origin indication of the primary ingredient of a food.
British Retail Consortium (1 May 2019) Guidance on Commission Implementing Regulation (EU) 2018/775 of 28 May 2018 on the provision of food information to consumers, as regards the rules for indicating the country of origin or place of provenance of the primary ingredient of a food.
- This document is not intended as a comprehensive guide, but a top-line summary for information.
Primary ingredients legislation white paper
Click below to download a copy of this white paper in PDF format
For more information, please contact:
Allergen labelling and other legislation issues:
Preventing and monitoring allergen cross-contamination:







