Predictive modelling to determine shelf-life – what can it tell us?
16 February 2023 | Linda Everis, Section Lead
How do we define shelf-life? It’s typically the time after production during which a food or drink product remains acceptable for consumption. A straightforward definition, right? But the food and drink industry faces many hurdles when dealing with the complex task of setting shelf-life testing protocols.
From manufacture to consumer storage, products can encounter a range of fluctuating temperatures as they are passed from one stage of the cold chain to the next. Determining what these temperatures are and the time spent at them is a challenge. But it must be done to deliver a relevant and realistic shelf-life protocol.
Retailers have often developed their own company-specific shelf-life protocols for manufacturers to follow. When developing them, they ask questions such as, “How long will the product be out of chill when purchased?” and “What temperatures will the product be stored at during purchase and in the consumer’s fridge?”. Often, protocols can differ between retailers, meaning a manufacturer may end up supplying similar products to different customers while following different test protocols.
This leaves us to question whether these differences in shelf-life protocol significantly impact a product’s assigned shelf-life. Will spoilage microorganisms behave in the same way? How much faster may they grow and spoil the product? How much of a potential food safety risk is there? Predictive modelling helps us to answer these questions.
Download the complete Maximising Shelf-life eBook, for free, to unlock the tools for measuring and maximising the shelf-life of your food and drink products whilst ensuring their safety and quality.

What is predictive modelling?
Predictive modelling is a tool used to assess the survival and/or growth of a microorganism under a range of temperature, pH and water activity (aw) conditions. The models are generated using computer-based software packages and can be used to quickly assess the effect of different storage regimes on the microbiological levels in food and drink products. It’s also extremely useful at the product development stage, allowing a quick evaluation of the impact of recipe changes – for example, the effect of salt reduction on Listeria. Overall, it can be used to help determine the likely shelf-life of products from both a quality and a safety perspective.
Predictive modelling can be used alongside shelf-life testing of real-life samples, for example using challenge testing. Challenge testing involves deliberately inoculating a product with relevant microorganisms and assessing their growth over time. This approach considers all factors that may influence growth. Predictive modelling, however, will usually only consider three factors – pH, aw and temperature.
Factors not accounted for in predictive modelling:
- natural antimicrobials that may be present (for example, allicin in garlic)
- differences in structure across a food sample
- change of aw and pH over time, and
- competing organisms
How does predictive modelling work?
Laboratory generated microbial growth curves are used to produce the models. Microbiological growth media (broths) with different intrinsic parameters (for example, varying pH and salt levels) are inoculated with the relevant microorganism (or cocktail of microorganisms) and incubated at a range of temperatures. Mathematical equations are then applied to the data and used to construct models that allow us to predict how the microorganisms are likely to grow in conditions similar to that of a particular product. As a result, we can then use this data to predict what kind of shelf-life this product will have.
What predictive modelling can tell us about shelf-life protocols
We used this technique to investigate how seven different protocols, for setting shelf-life, showed variations in the growth of different microorganisms. Cooked ham and raw meat were chosen for the study. The goal was to understand how slight differences in storage protocols impacted the outcomes of shelf-life testing.
To do this, two approaches were taken. One to assess the product’s safety (by measuring the growth of two pathogenic species: Listeria and C. botulinum) and the other to assess the product’s quality (by measuring the level of the spoilage microorganism Enterobacteriaceae). The ‘end of shelf-life’ was defined in relation to levels of each of these microorganisms – an increase of 0.5 log cfu/g for C. botulinum or from 10 to 100 cfu/g for Listeria (at this point, the product was considered as unsafe), or reaching 106 cfu/g for Enterobacteriaceae (at this point, the product was considered as spoilt).
What did we find?
The results highlighted considerable differences in the growth potential of microorganisms depending on the storage protocol used. This would affect the setting of shelf-life for those products. This is significant, especially where food safety is concerned. Neurotoxin-producing C. botulinum showed this variation most clearly in the duration of the shelf-life that was predicted to be with the consumer, and therefore at the highest temperature of the cold chain. The protocols that modelled the product being with the consumer for the most time showed significant growth of this microorganism. In fact, the shelf-life was shortened by 14 days in the most extreme case compared to the protocol that hadn’t factored in as much time with the consumer.
[pH : 6.29 aw: 0.980]. Assumed Initial level: 10 cfu/g.
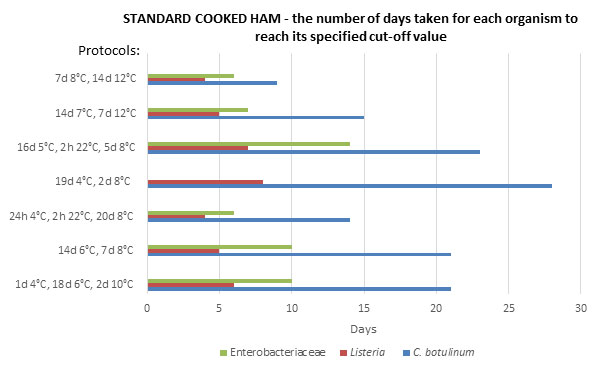
*106 cfu/g level not reached by Enterobacteriaceae for protocol ‘19d 4°C, 2d 8°C’.
Figure 1: Potentially achievable shelf-life in standard cooked ham – the number of days taken for each organism to reach its specified cut-off value
[pH : 5.66 aw: 0.986]. Assumed Initial level: 10 cfu/g.
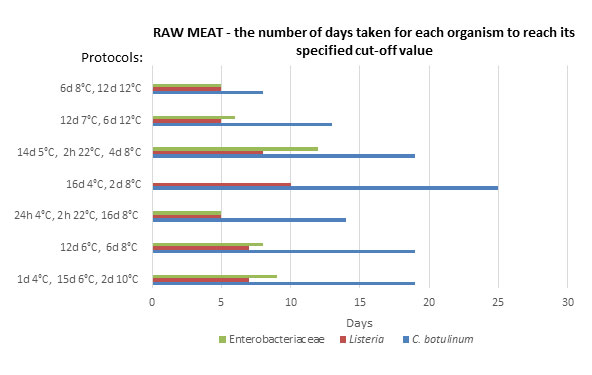
*106 cfu/g level not reached by Enterobacteriaceae for protocol ‘16d 4°C, 2d 8°C’
Figure 2: Potentially achievable shelf-life in raw meat – the number of days taken for each organism to reach its specified cut-off value
As these results have shown, following one storage protocol can lead to unsafe food two weeks earlier than another (when both are applied to the same product), so it does make you question which protocol is more appropriate and most realistic. The food and drink industry should be aware of these differences and think about how realistic their testing protocols are when all factors are taken into consideration.
We have produced a guideline document, Guideline 46 on setting the microbiological shelf-life of chilled foods for the industry. It discusses in detail what manufacturers and retailers must do to produce a shelf-life protocol that ensures the safety and quality of their products.
At Campden BRI, we can perform both challenge testing and predictive modelling as a practical service to establish the shelf-life of food and drink products. There are specific benefits to each of these tests, but you can find out which is most suitable for your needs by getting in touch.

About Linda Everis
Linda has a wealth of knowledge, experience and expertise from her time in Microbiological Analytical Services and Microbiology Safety and Spoilage. She joined Campden BRI in 1995 as a Senior Technician in the Microbiological Analytical Services group having graduated from the University of Wales Aberystwyth with a BSc in Biology.
Alongside the expert support that Linda provides to our members and clients, Linda conducts project work and research, and has been author and co-author of articles and papers for a variety of microbiological publications and Campden BRI reports.
Linda also lectures on shelf-life, challenge testing and predictive microbiology for our many training courses and seminars.
Maximising Shelf-life eBook
For more on measuring, setting and extending food and drink product shelf-life, download our ‘Maximising Shelf-life’ eBook today.
How can we help you?
If you’d like to find out more about how we can help, contact our Support Team.