
What effect does novel packaging have on microflora?
By Greg Jones and Lynneric Potter - 2 November 2020
Today’s food packaging landscape is driven by the desire to minimise environmental impact while maximising shelf-life and presenting products attractively to the consumer. Packaging manufacturers are rising to the challenge to achieve this, and there are a large number of novel materials now being presented to the food industry – often with claims of similar or better performance to traditional materials. Some manufacturers also claim that their packaging materials have an antimicrobial effect which can extend shelf-life. As promising as they may sound, how can these claims be proven?
Independently substantiating claims
A red meat processor, working with a materials developer, wanted to understand the impact of three novel films for potential packaging use on the microflora of red meat during retail shelf-life. The hypothesis being that the novel films would have an inhibitory effect on the growth of microbes, ultimately extending the shelf-life of the product. To understand clearly any microbial changes, the red meat processor approached us to conduct an independent study on the product packaged using the novel materials.
The beef had been aged for 21 days before packing as per standard practice. Three novel materials were used to determine if there would be a difference between formulations. These three novel films were tested against standard skin packaging, vacuum packaging and modified atmosphere packaging (MAP) – packaging materials commonly used in the food industry.
Microflora methods
The analysis of microflora has traditionally relied on counting the numbers of colonies growing on agar media which is incubated at a set temperature for a set amount of time: a cornerstone method that’s formed the basis of microbiology for over a century. In contrast, new techniques reliant on sequencing genetic material are less restricted. They do not select only for the organisms that can grow on the selected media for the selected time at the selected temperature. The new methods can detect genetic material from organisms that would not usually grow in the laboratory and thus provide a more comprehensive picture of the total microbial population. Sequencing-based approaches also allow a finer resolution of groups of organisms which are difficult to sub-divide using culture alone.
To determine whether or not the packaging had made a difference to the samples’ microflora, the beef samples were studied using advanced microbial profiling: a DNA sequencing-based analysis. This technique allows huge numbers of different individual DNA sequences to be read at any one time and was performed using the Thermo Scientific™ Ion Chef™ Food Protection Instrument and Ion GeneStudio™ Food Protection NGS System.
What did we find?
Results from the sequencing showed that the novel packaging had no detectable effect on the microflora of the products, and that the only packaging type that had any effect on microflora was the MAP format. Figure 1 illustrates this by representing each detected organism as a specific colour. MAP was the only form of packaging that prevented the growth of Lactococcus..
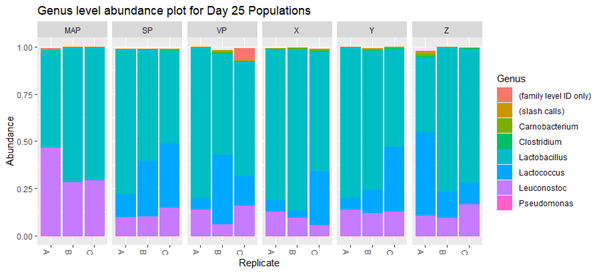
The results allowed the red meat processor to conclude that the novel film had the same effect as the current close-contact films, enabling the food business to make future decisions on whether to use the novel film based on sound evidence.
Have you considered using an alternative packaging material but not sure on how effective it is? We have a range of packaging analysis and testing facilities and can conduct packaging investigations including microbiological, microscopic, chemical, sensory and migration and taint analysis and can advise on legal compliance. Get in touch to find out how we can help you.
Support Team
+44(0)1386 842291
support@campdenbri.co.uk

About Lynneric Potter
Lynneric has worked at Campden BRI since 1999, and has spent the majority of this time specialising in all things packaging, including supporting members and clients, managing contract projects, and conducting research.

